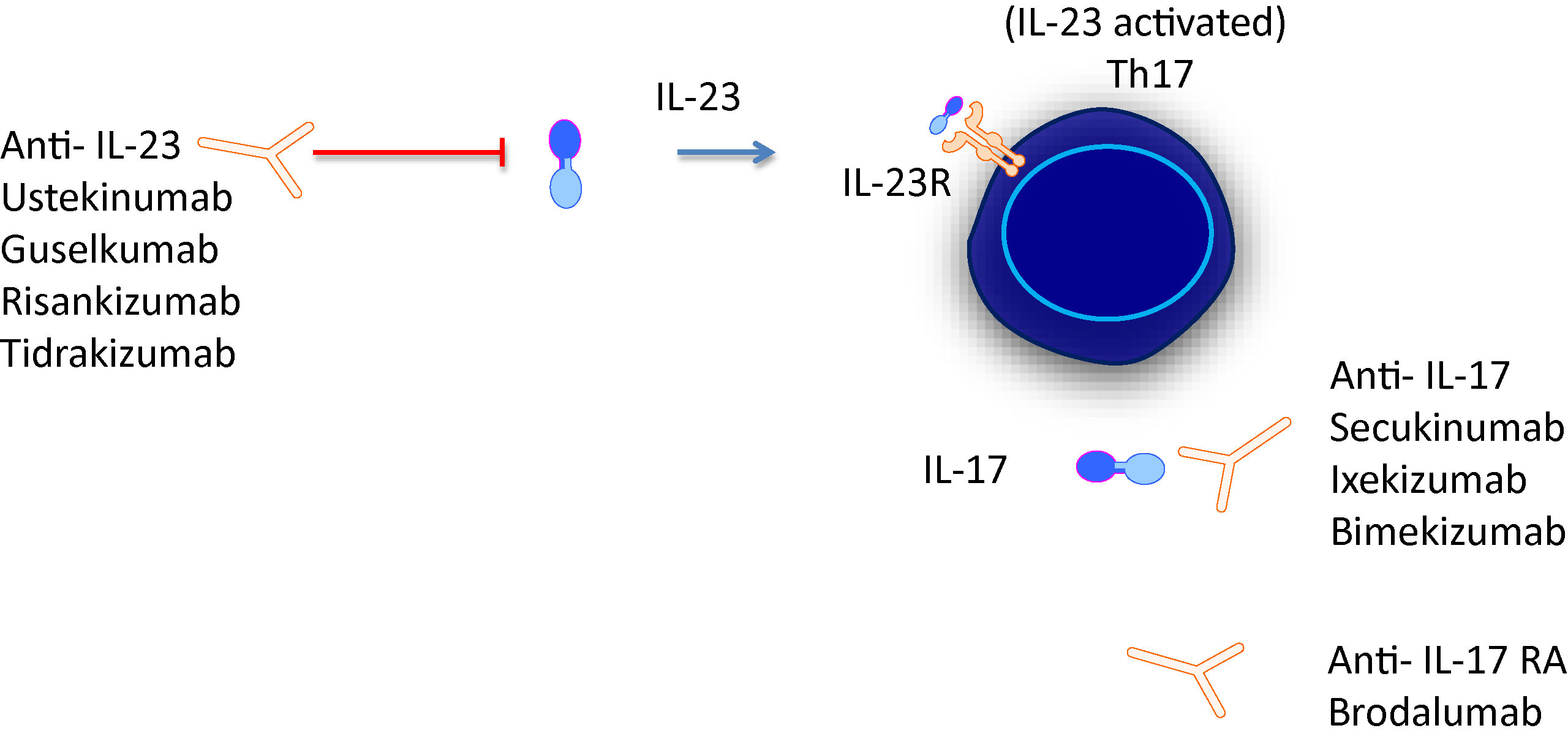
Therapies such as ustekinumab and guselkumab inhibit IL23 Ustekinumab targets the p40 subunit common to both IL23 and IL12 while guselkumab targets the p19 subunit found in IL23 IL17 subtypes trigger downstream inflammation in psoriasis Various drugs designed to interfere with the IL12–T H 1 and IL23–T H 17 pathways, such as IL23specific antibodies, oral peptide inhibitors ofIL23 inhibitor risankizumab induces remission in Phase II study in patients with moderatetosevere Crohn's disease Print INGELHEIM, Germany & NORTH CHICAGO, IllTuesday AETOS Wire After 12 weeks, approximately twice as many

Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut
Il-23 inhibitor crohn's
Il-23 inhibitor crohn's-Likewise, in a study of an IL23 inhibitor in Crohn's disease, patients with higher baseline serum concentrations of IL22, a cytokine induced by IL23, were more likely to respond More research is needed on biomarkersIL23 inhibitors have shown to be effective to both psoriasis and CD Case presentation Fortyone year old Chinese male patient who came to the hospital for psoriasis, developed severe gastrointestinal symptoms after using an IL17 inhibitor, and




Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases
Introduction Blockade of interleukin (IL)12 and IL23 is a novel therapeutic target for inflammatory bowel disease (IBD) The monoclonal antibody targeting the shared p40 subunit of IL12 and IL23, namely ustekinumab, has been approved for Crohn's disease (CD) and has demonstrated promising results in the treatment of ulcerative colitis IL23 inhibitors are a type of biologic that can treat moderatetosevere psoriasis Learn more about these medications, including how they work, the possible side effects, and more There is second IL23 inhibitor still in Phase III trials for AS (not Risankizumab) and it is not doing well either apparently In Stelara crohns dose is 90 mg every 48 weeks Similar studies done on AS lower PsA dosing
LEXINGTON, Mass, Dec 23 /PRNewswire/ Synta Pharmaceuticals, a biopharmaceutical company discovering, developing, and commercializing small molecule drugs to treat severe medical conditions, announced today that it will focus its clinical efforts in 06 on the company's oral IL12/ IL23 inhibitor, STA5326, currently in phase 2b development for Crohn'sFuss IJ, Becker C, Yang Z, et al Both IL12p70 and IL23 are synthesized during active Crohn's disease and are downregulated by treatment with antiIL12 p40 monoclonal antibody Inflamm Bowel Dis 06; IL23 Inhibitor Risankizumab Induces Remission in Phase II Study in Patients with ModeratetoSevere Crohn's Disease After 12 weeks, approximately twice as many patients with moderatetosevere Crohn's disease, the majority of whom had previously failed treatment with one or more TNF antagonists, achieved clinical remission with risankizumab compared with placebo(1)
New phase 3 data show the IL23 inhibitor is associated with clinical remission and endoscopic response Data from 2 phase 3 induction studies of risankizumab (SKYRIZI) demonstrate that the interleukin23 (IL23) inhibitor can lead to clinical improvements in patients with moderate to severe Crohn's disease Clinical trials have assessed the therapeutic effect of an IL12/IL23 inhibitor (ustekinumab), demonstrating rapid clinical effect with a safety profile Further studies are needed to elucidate its potential role as firstline therapy in Crohn's disease IL23 is produced locally in the intestine and the upregulation of IL23 and its receptor is a primary step underpinning intestinal inflammation Since IBD represents a local inflammation of the intestinal tissue, an ideal therapeutic would act from the luminal side to achieve high drug concentrations in diseased tissue (enhanced efficacy




Biological Agents Evaluated In Inflammatory Bowel Disease Download Table




Risankizumab An Il 23 Inhibitor For Ankylosing Spondylitis Results Of A Randomised Double Blind Placebo Controlled Proof Of Concept Dose Finding Phase 2 Study Annals Of The Rheumatic Diseases
Crohn's could be an important indication for Lilly, as mirikizumab is very much playing catchup in the IL23 inhibitor category when it comes to psoriasis First to market was Johnson & Johnson's dual IL12/IL23 inhibitor Stelara (ustekinumab) for psoriasis, which rapidly achieved blockbuster status with addon indications in psoriatic arthritis and Crohn's and made B Feagan et al Efficacy and safety of induction therapy with the selective IL23 inhibitor risankizumab (BI ), in patients with moderatetosevere Crohn's disease Results of a randomized AbbVie Receives Orphan Drug Designation for Investigational IL23 Inhibitor Risankizumab from the US Food and Drug Administration for the Treatment of Pediatric Patients with Crohn's Disease




Il 23 Producing Il 10ra Deficient Gut Macrophages Elicit An Il 22 Driven Proinflammatory Epithelial Cell Response
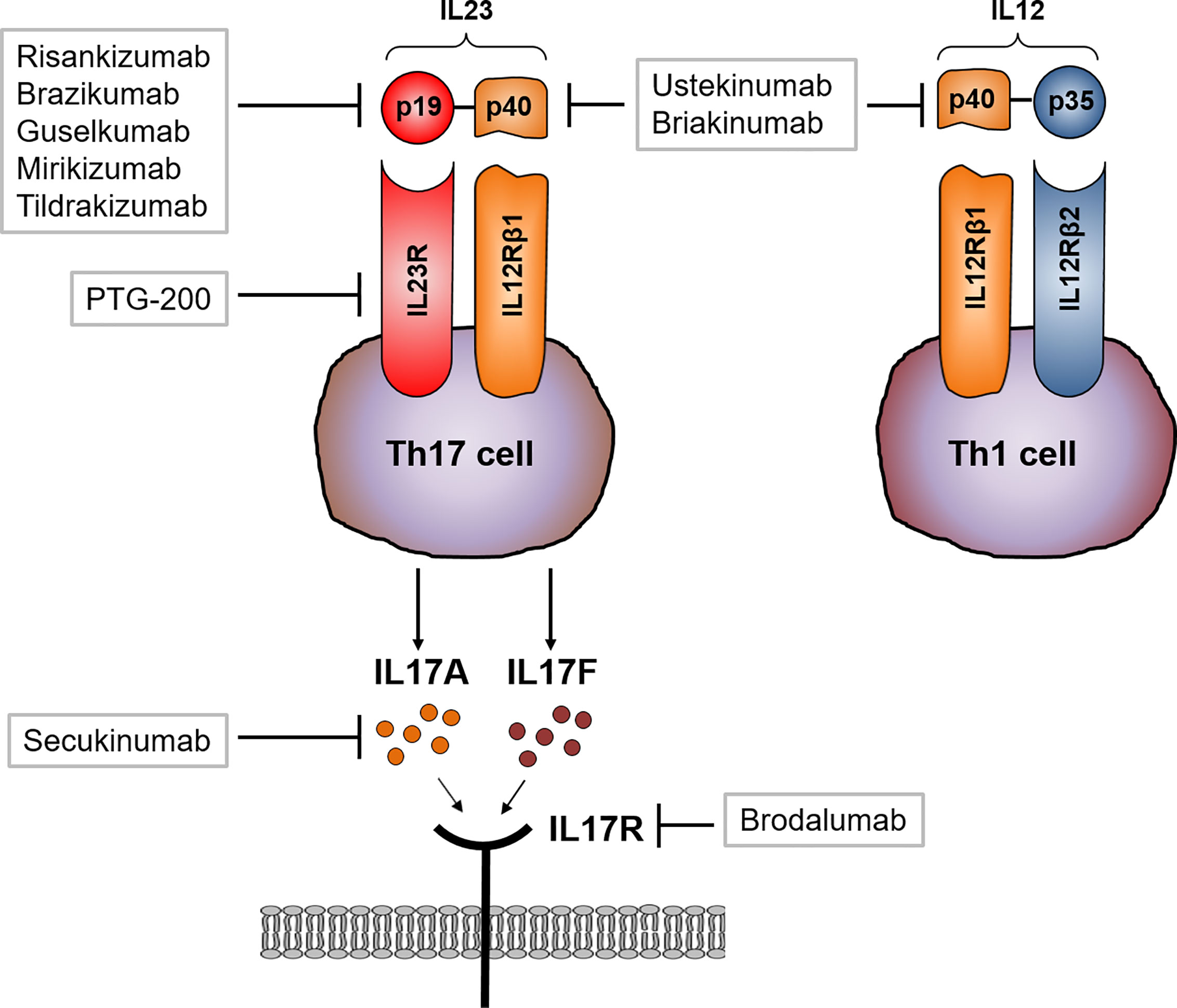



Frontiers Role Of The Il23 Il17 Pathway In Crohn S Disease Immunology
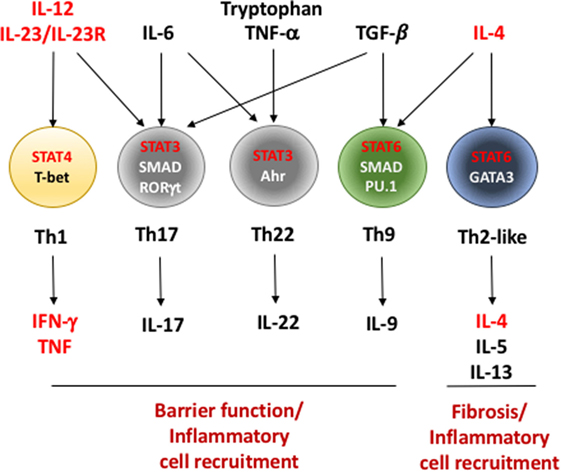
The company reported positive Phase II results and will move its potential fourthtomarket IL23 inhibitor into Phase III in Crohn's disease later this year Mirikizumab – in Phase III for psoriasis and ulcerative colitis – may be the second IL23 drug for an IBD indication B Feagan et al Efficacy and safety of induction therapy with the selective IL23 inhibitor risankizumab (BI ), in patients with moderatetosevere Crohn's disease Results of a randomized, doubleblind, placebocontrolled Phase II study Digestive Disease Week, San Diego, USA, 2124th May 16 Abstract ID The interleukin (IL)12 family of cytokines, including IL12 and IL 23, play an important role in driving aberrant Th1 and Th17 immune responses in patients with Crohn's disease (CD) Targeting this pathway has opened new avenues for therapeutic intervention




Pdf Population Pharmacokinetics Of The Interleukin 23 Inhibitor Risankizumab In Subjects With Psoriasis And Crohn S Disease Analyses Of Phase I And Ii Trials




Interleukin 23 In Ibd Pathogenesis Intechopen
Crohn's disease and ulcerative colitis are the two extreme phenotypes of the spectrum of inflammatory bowel diseases (IBDs) These are chronic inhibitors of interleukin (IL) 12/IL23 Unfortunately, primary nonresponse is observed in –30% of patients, and another 30% of In Crohn's disease, IL23 inhibition with p40 (ustekinumab) and p19 (risankizumab) inhibitors has demonstrated efficacy in phase II/III studies15 16 In contrast, a phase II study of IL17A inhibition with secukinumab did not meet its primary outcome and a phase II study of brodalumab, an IL17RA inhibitor, was prematurely stopped, with Crohn's could be an important indication for Lilly, as mirikizumab is very much playing catchup in the IL23 inhibitor category when it comes to psoriasis First to market was Johnson & Johnson's dual IL12/IL23 inhibitor Stelara (ustekinumab) for psoriasis, which rapidly achieved blockbuster status with addon indications in psoriatic




Novel Pharmacological Approaches For Inflammatory Bowel Disease Targeting Key Intracellular Pathways And The Il 23 Il 17 Axis
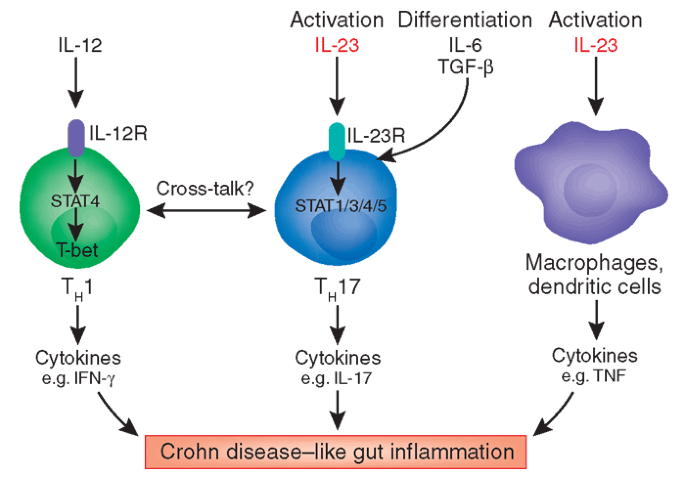



Il 23 A Master Regulator In Crohn Disease Nature Medicine
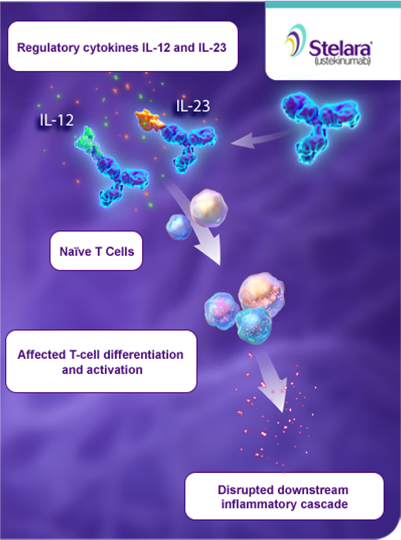
Risankizumab is a humanized monoclonal antibody targeting the p19 subunit of interleukin23, and is being developed for Crohn's disease Other IL23 inhibitors are currently under study for inflammatory bowel disease, psoriasis, psoriatic arthritis, and spondyloarthritisIntroduction Blockers of IL12/23, as well as specific blockers of IL23, have been investigated as options for medical therapy in inflammatory bowel disease These biological agents include ustekinumab the first agent of this pharmacological class which has shown clinical efficacy in psoriasis, psoriatic arthritis, and moderatetosevere Crohn's disease (CD) and otherAnd ustekinumab, a biologic that targets cytokines interleukin12 and interleukin23 (IL12 and IL23), has been approved for Crohn's disease treatment According to Michael F Picco, MD, PhD , the approach to maximizing the effectiveness of these
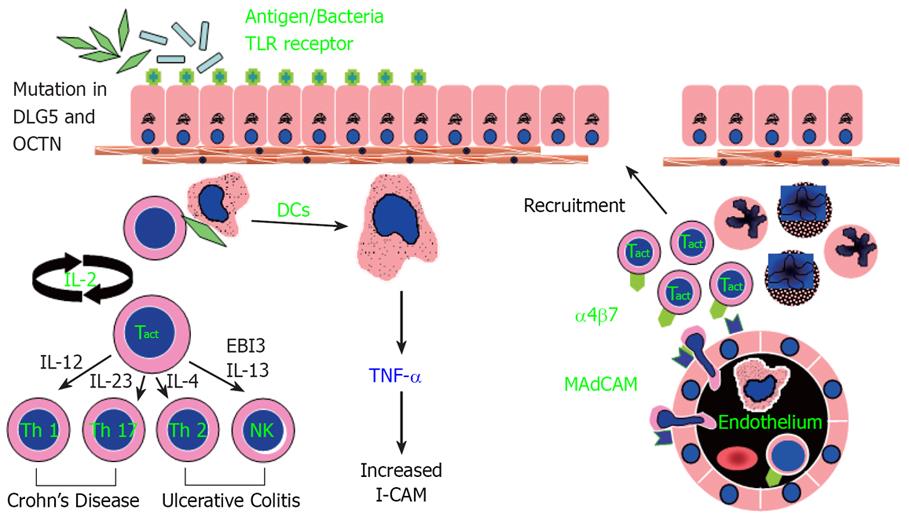



Immunopathogenesis Of Inflammatory Bowel Disease




Differential Roles For Interleukin 23 And Interleukin 17 In Intestinal Immunoregulation Sciencedirect
The IL23 inhibitors work by combatting the IL23 cytokine, which stimulates Th17 cells to produce the inflammatory IL17A, IL17F, and IL22 cytokines IL23 is found in the skin of patients with psoriasis, in the bowel wall of patients with inflammatory bowel diseases like Crohn's, and in the synovial membrane of patients with rheumatoidBS A handful of agents have been, or are currently being, looked at as inhibitors of either interleukin (IL)12 and 23 or just IL23 Ustekinumab (Stelara, Janssen), which was recently approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe Crohn's disease, is an antibody directed against the p40 subunit shared by IL12 and 23The interleukin (IL)12 family of cytokines, including IL12 and IL 23, play an important role in driving aberrant Th1 and Th17 immune responses in patients with Crohn's disease (CD) Targeting this pathway has opened new avenues for therapeutic intervention
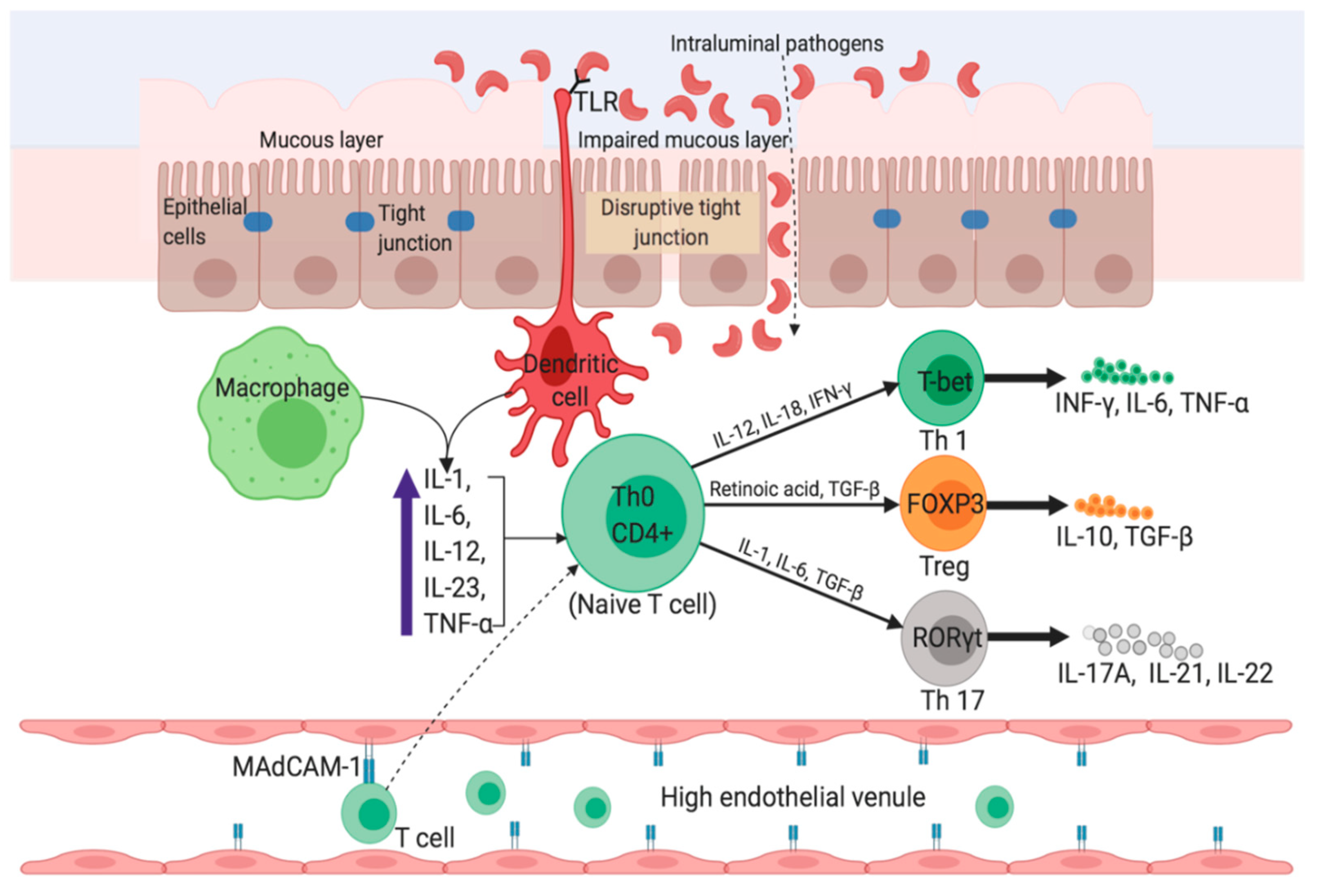



Jcm Free Full Text Revisiting Inflammatory Bowel Disease Pathology Treatments Challenges And Emerging Therapeutics Including Drug Leads From Natural Products Html




Shifting The Focus The Primary Role Of Il 23 In Psoriasis And Other Inflammatory Disorders Gooderham 18 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library
About a year and a half after presenting positive topline results in Crohn's disease, Eli Lilly gave its experimental IL23 antiinflammatory mirikizumab a * Abbvie receives Orphan Drug Designation for investigational IL23 inhibitor Risankizumab from the US Food and Drug Administration for the treatment of pediatric patients with Crohn's Disease SKYRIZI is an interleukin23 (IL23) inhibitor that selectively blocks IL23 by binding to its p19 subunit 15,16 IL23, a cytokine involved in inflammatory processes, is thought to be linked to a number of chronic immunemediated diseases, including Crohn's disease 15,16 In April 19, SKYRIZI received US Food and Drug Administration




Shifting The Focus The Primary Role Of Il 23 In Psoriasis And Other Inflammatory Disorders Gooderham 18 Journal Of The European Academy Of Dermatology And Venereology Wiley Online Library




Classes Of Biologics For The Treatment Of Ibd Alpco
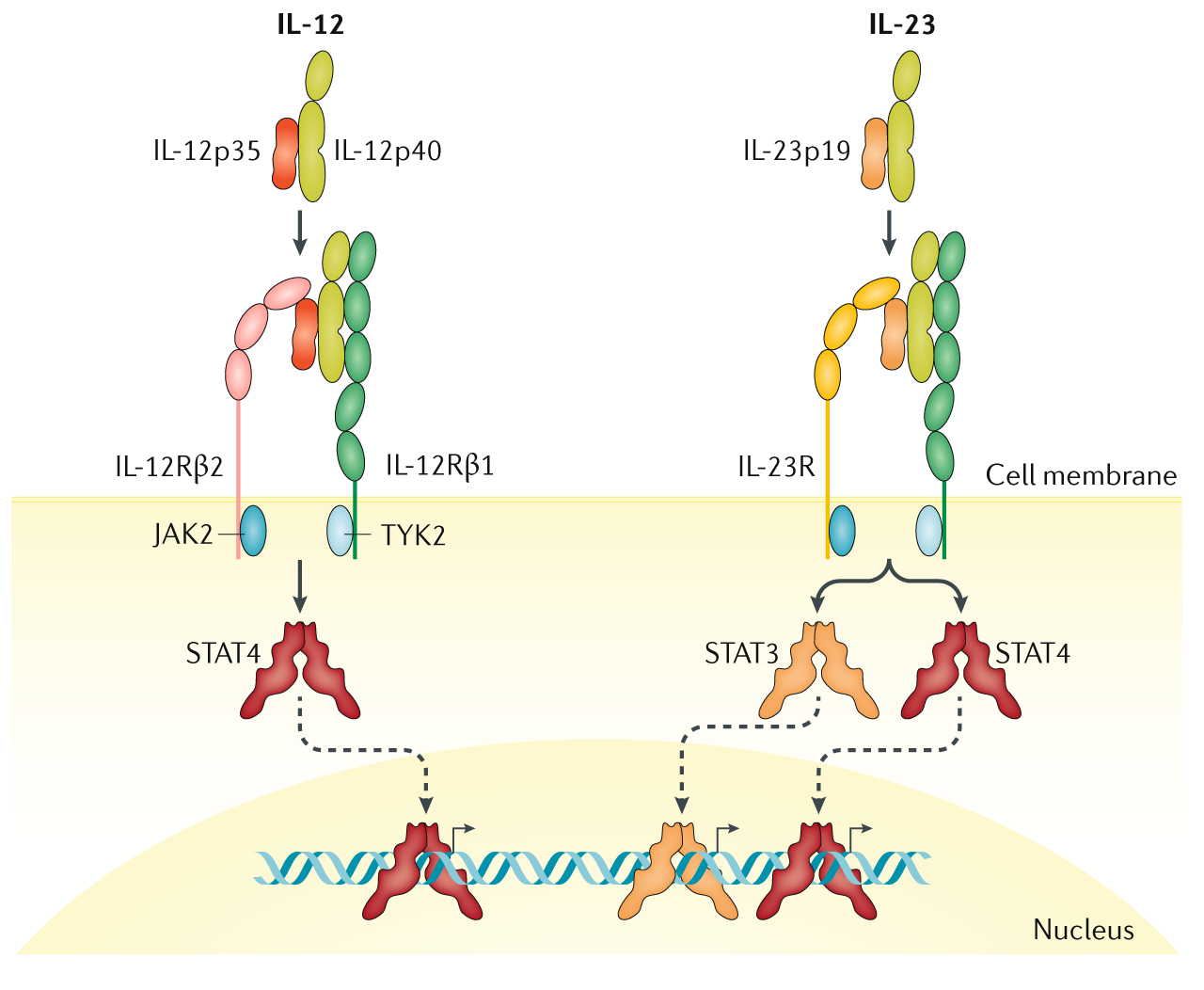
Selective IL23 inhibition has been touted to be the next great advance in the treatment of inflammatory bowel and psoriatic disease The annual Digestive Disease Week (DDW) conference in San Diego reported early reults from a proofofconcept, Phase II study, wherein risankizumab (antiIL23) was shown to be more effective than placebo in patients withIn the class of anticytokine agents, an antiIL12/IL23 (antip40) monoclonal antibody has entered clinical practice in Crohn's disease (CD) and demonstrated efficacy in clinical trials for ulcerative colitis (UC), and several selective antiIL23 agents (antip19) have shown efficacy and are being further investigatedFunction The protein encoded by this gene is a subunit of the receptor for IL23This protein pairs with the receptor molecule IL12Rβ1 (), together forming the IL23 receptor complex, and both are required for IL23 signalingThis protein associates constitutively with Janus kinase 2 (), and also binds to transcription activator STAT3 in a liganddependent manner



Frontiers Mini Review New Treatments In Psoriatic Arthritis Focus On The Il 23 17 Axis Pharmacology



Frontiers Effector T Helper Cell Subsets In Inflammatory Bowel Diseases Immunology
Interleukin23 (IL23) inhibitors are an important new class of drugs for the treatment of Crohn disease (CD) and ulcerative colitis (UC), both common causes of inflammation of the digestive tract Johnson & Johnson's Stelara (ustekinumab) is the only IL23 inhibitor currently approved to treat moderatetosevere CD and UC in the United States Ustekinumab, an approved antiIL23/antiIL12 mAb that binds to the p40 subunit of IL23 (shared subunit of IL12 and IL23), has been shown to be efficacious in treating psoriasis, psoriatic arthritis, and Crohn's disease Other IL23 blockers include guselkumab, recently approved for treating psoriasis, and tildrakizumab, which is being * IL23 inhibitor risankizumab induces remission in phase ii study in patients with moderatetosevere crohn's disease




Small Molecule Oral Targeted Therapies In Ulcerative Colitis The Lancet Gastroenterology Hepatology



1
TNFα Inhibitor IV Crohn's disease Ulcerative colitis Infliximabqbtx Ixifi TNFα Inhibitor IV Crohn's disease Ulcerative colitis Natalizumab Tysabri α4 integrin inhibitor IV Crohn's Disease 11/1104/ Risankizumabrzaa Skyrizi IL23 Inhibitor SC Plaque psoriasis b 04/2319"IL23" to identify controlled clinical trials published up to June 17,16, with no start date restrictions The integrin inhibitor vedolizumab has been approved for Crohn's Interleukin23 receptor gene polymorphisms are associated with susceptibility to Crohn's disease, and interleukin23 is a key regulator of the Thelper17 cell B Feagan et al Efficacy and safety of induction therapy with the selective IL23 inhibitor risankizumab (BI ), in patients with moderatetosevere Crohn's disease Results of a randomized




Ustekinumab Binds To The P40 Subunit Of Il 12 And Il 23 Preventing Download Scientific Diagram




Crohn S Disease Market Will Experience Major Growth During Next Decade
Interleukin‐12 (IL‐12) and interleukin‐23 (IL‐23) are inflammatory cytokines linked to the Th‐1 and Th‐17 phenotypes associated with Crohn's disease (CD) We investigated the activity and safety of apilimod mesylate (formerly STA‐5326), an oral IL‐12 and IL‐23 inhibitor, in patients with active CDPublished literature implicates both the IL12 and IL23 pathways in the pathogenesis of systemic lupus erythematosus Ustekinumab is a monoclonal antiIL12 and IL23 antibody that is approved for use in adults with psoriatic arthritis and Crohn's disease and adult and adolescent patients with plaque psoriasis Added value of this study To Therefore, selective blockade of interleukin23 via inhibition of p19 might be a viable therapeutic approach in Crohn's disease Induction therapy with the selective interleukin23 inhibitor risankizumab in patients with moderatetosevere Crohn's disease a randomised, doubleblind, placebocontrolled phase 2 study The Lancet



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology



Stelara Ustekinumab Mechanism Of Action Plaque Psoriasis




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




Emerging Treatments For Crohn S Disease Emj Reviews




Jci Insight Therapeutic Manipulation Of Innate Lymphoid Cells




Anti Interleukin 12 Antibody For Active Crohn S Disease Nejm
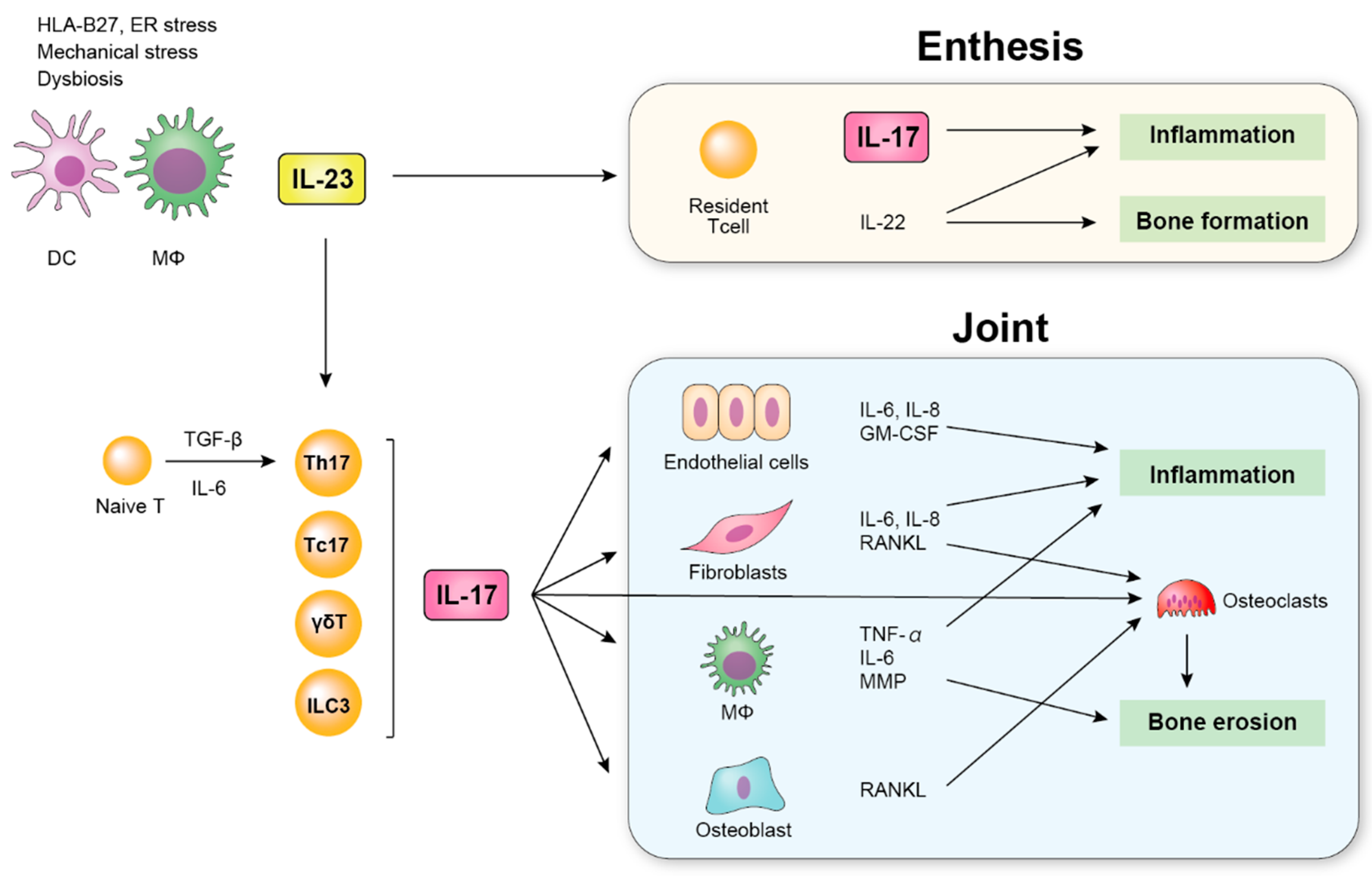



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html




The Proinflammatory Effect Of Prostaglandin E2 In Experimental Inflammatory Bowel Disease Is Mediated Through The Il 23 Il 17 Axis The Journal Of Immunology
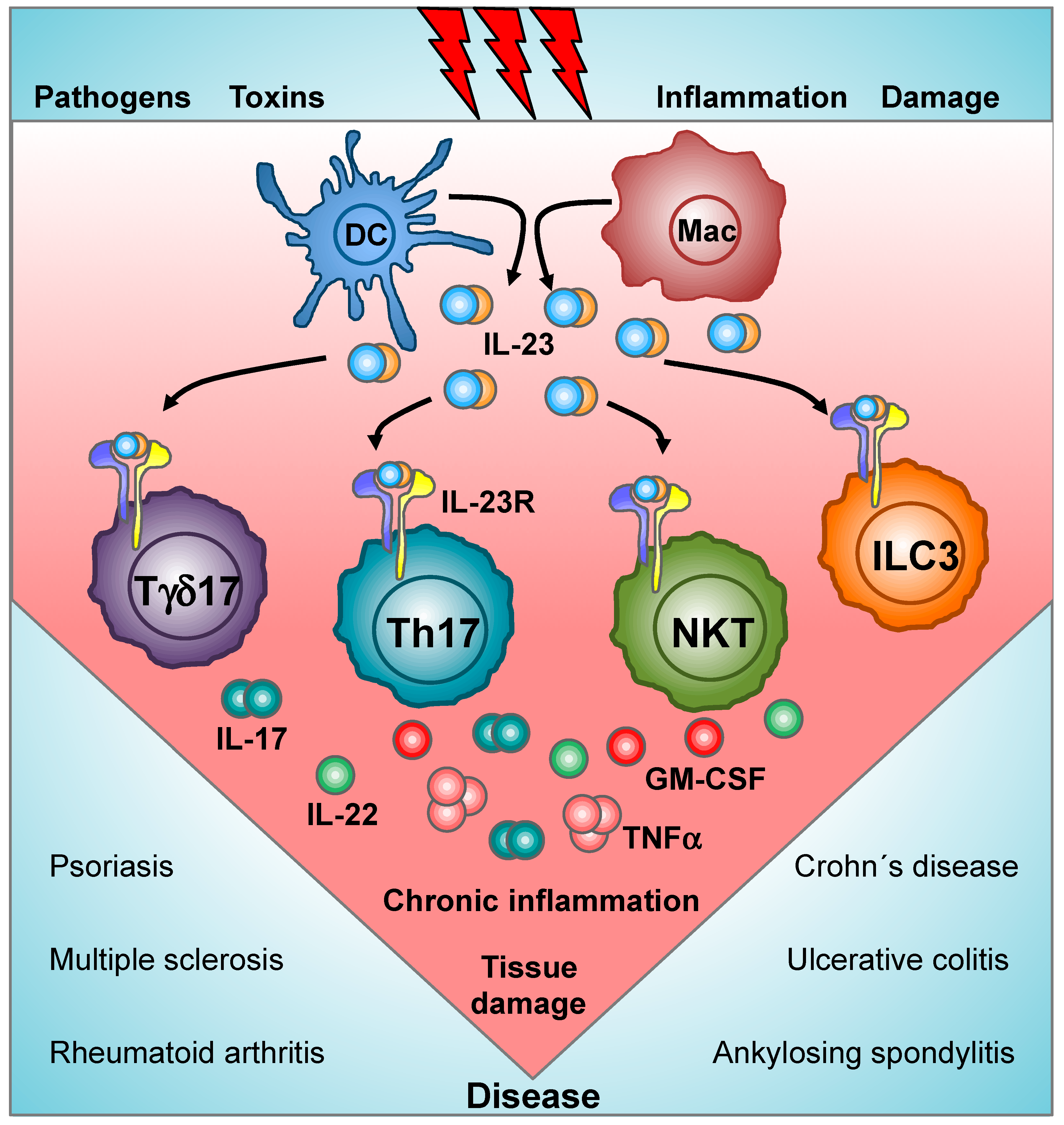



Cells Free Full Text Decoding Il 23 Signaling Cascade For New Therapeutic Opportunities Html
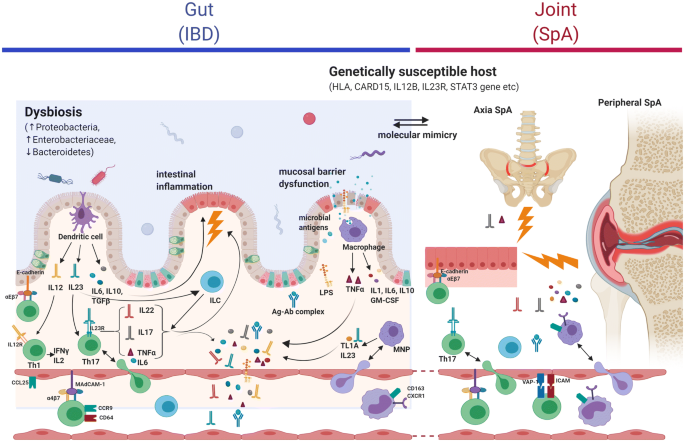



Defining The Phenotype Pathogenesis And Treatment Of Crohn S Disease Associated Spondyloarthritis Springerlink




The Il23 Axis Plays A Key Role In The Pathogenesis Of Ibd Gut




Immunological Pathogenesis Of Inflammatory Bowel Disease




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt



1




An Overview Of Novel And Emerging Therapies For Inflammatory Bowel Disease European Medical Journal




An Overview Of Novel And Emerging Therapies For Inflammatory Bowel Disease European Medical Journal



Inflammatory Bowel Disease Arthritis Rheumatism




The Currently Approved And Available Ibd Therapies Include Four Download Scientific Diagram




Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases




Can Il 23 Be A Good Target For Ulcerative Colitis Sciencedirect
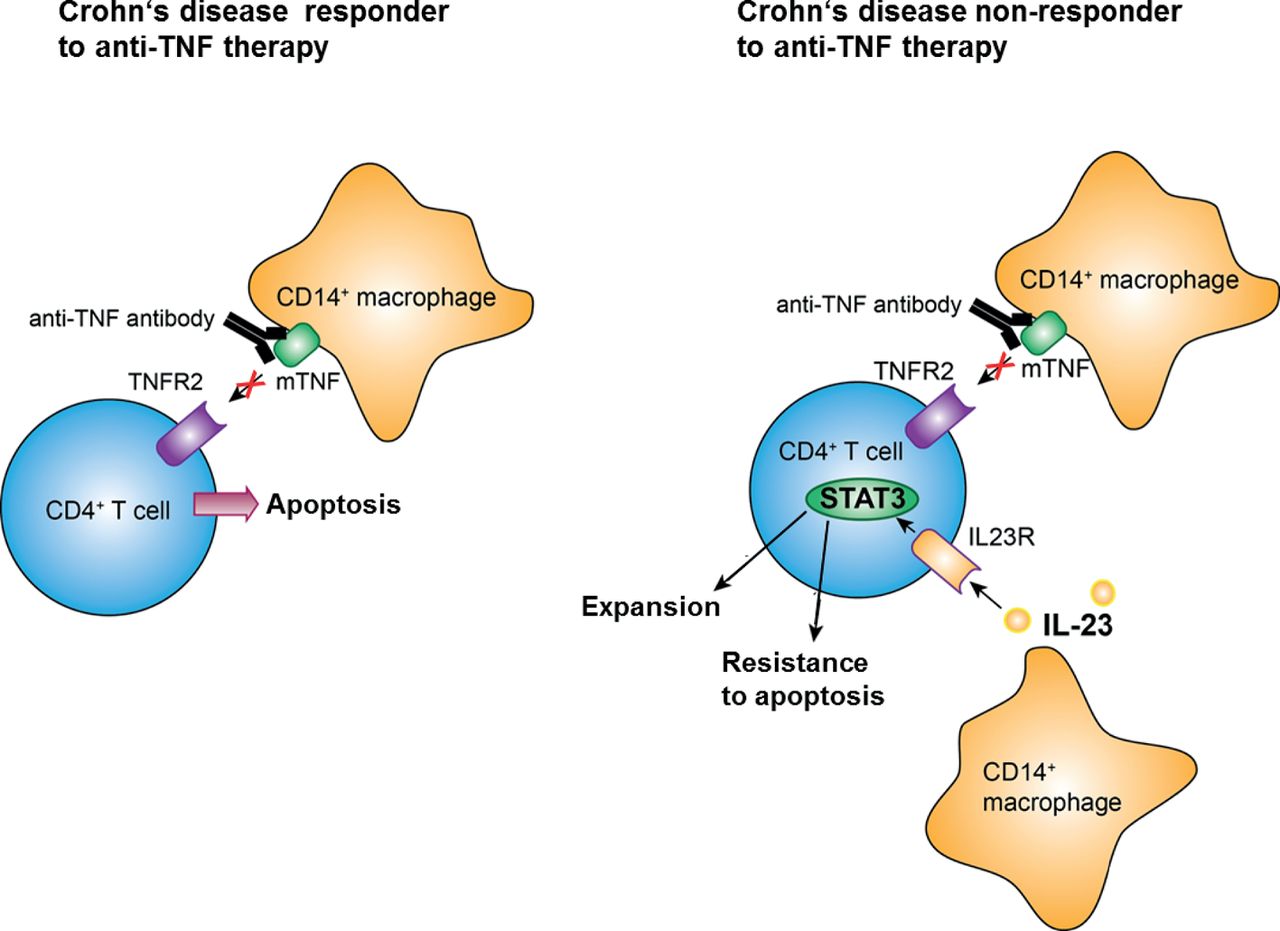



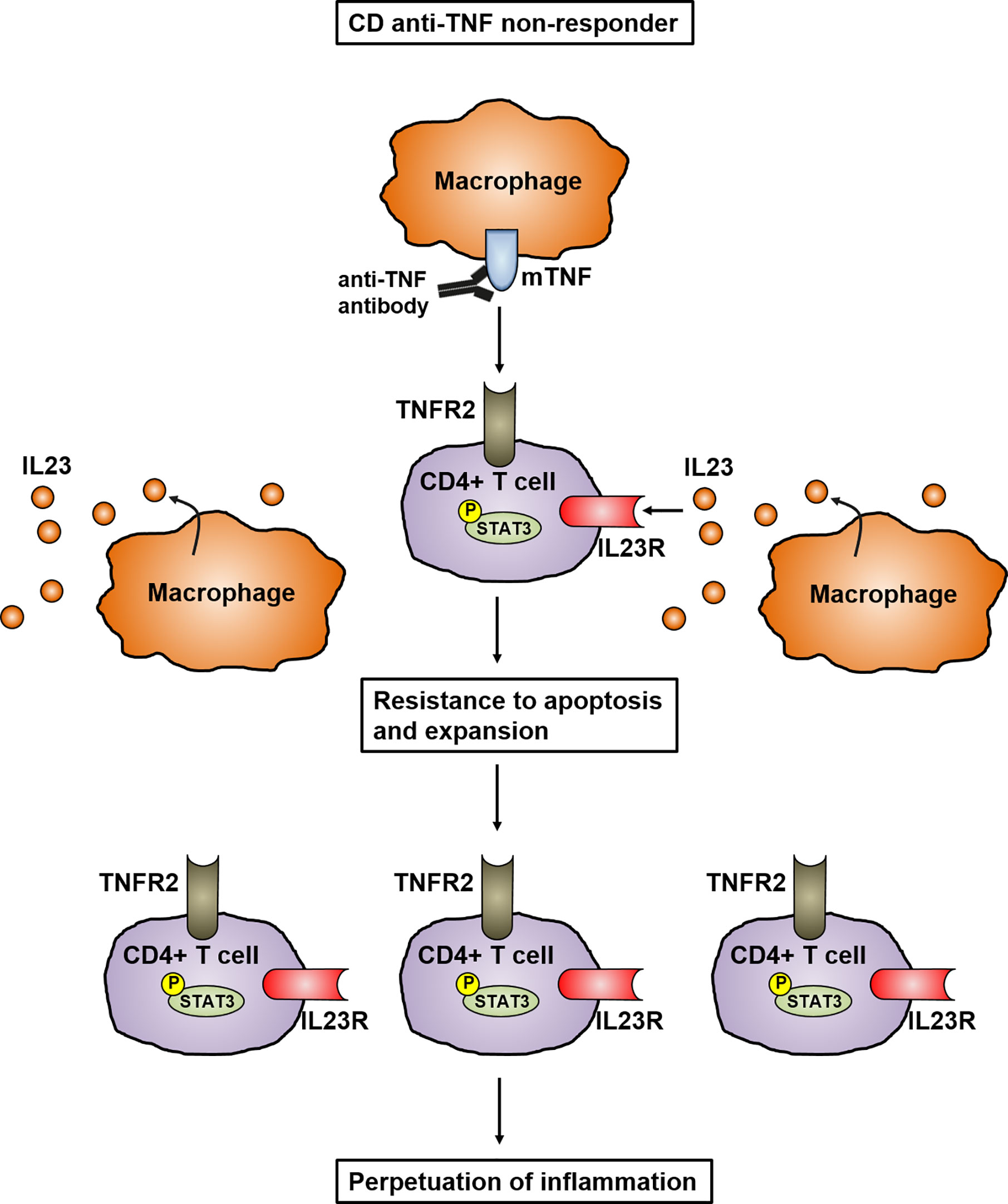
Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut
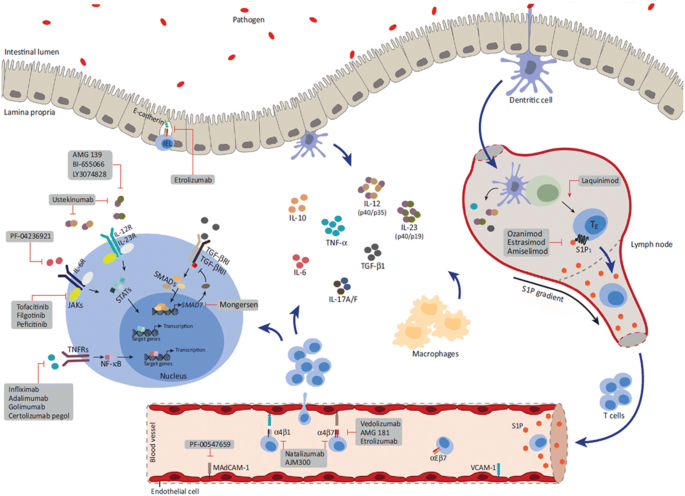



Emerging Therapies For Inflammatory Bowel Disease Springerlink




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt
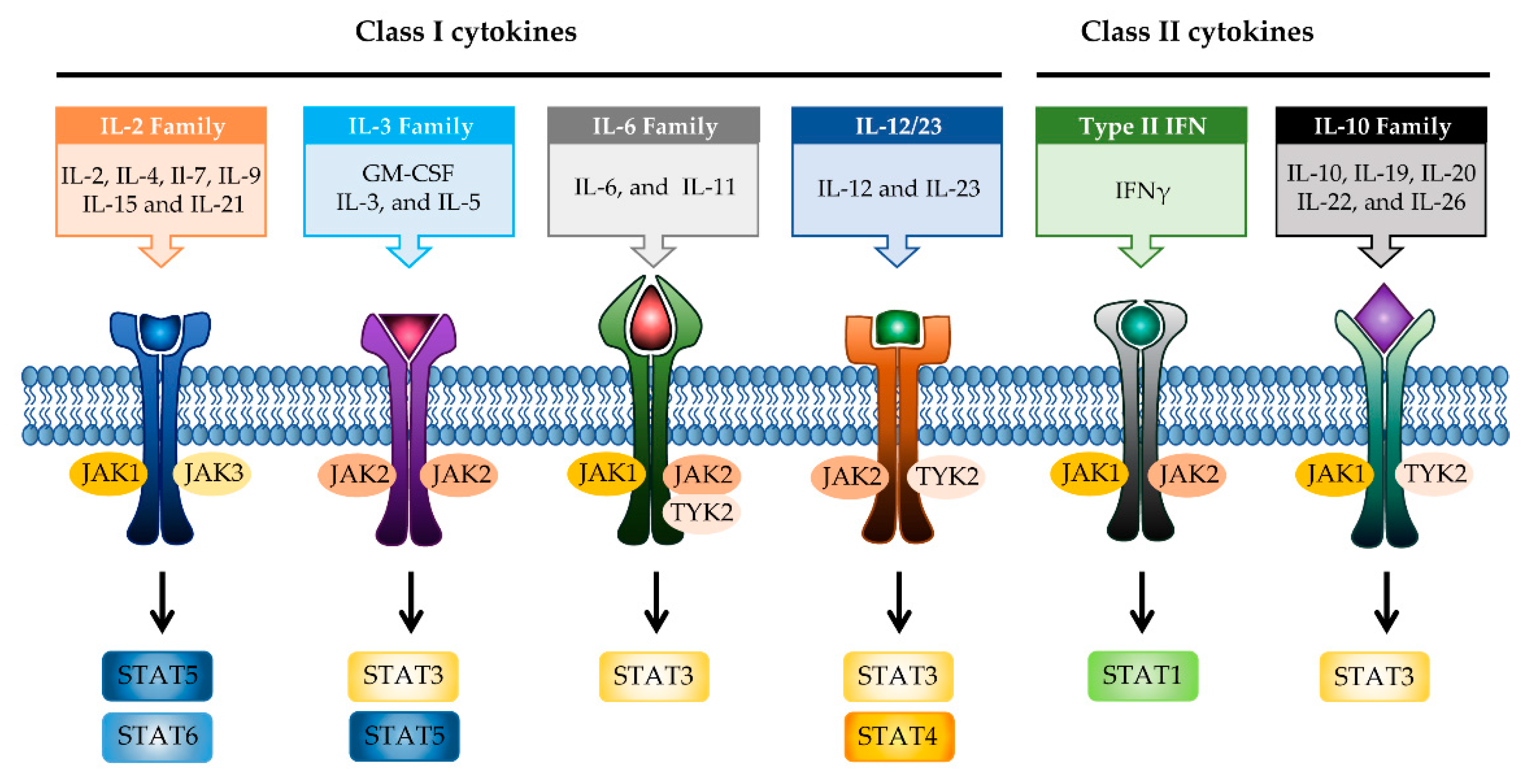



Molecules Free Full Text Phytochemicals Targeting Jak Stat Pathways In Inflammatory Bowel Disease Insights From Animal Models Html




Interleukin Il 12 And Il 23 Structure And Receptors Notes The Il 12 Download Scientific Diagram




Upadacitinib For Crohn S Disease And Ulcerative Colitis Treatment Hitting The Selective Jakpot Gastroenterology
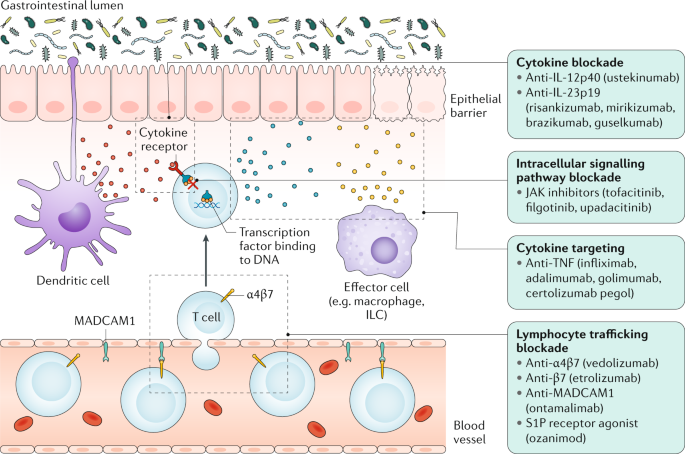



Interrogating Host Immunity To Predict Treatment Response In Inflammatory Bowel Disease Nature Reviews Gastroenterology Hepatology
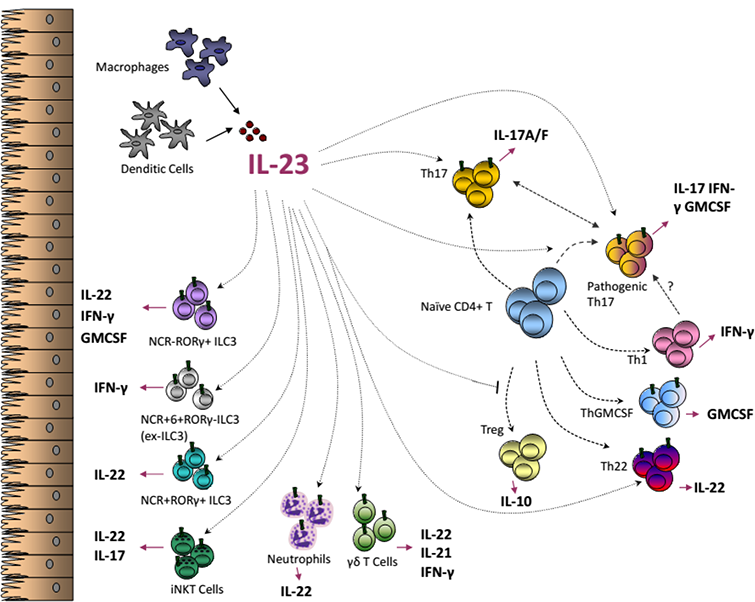



Interleukin 23 In Ibd Pathogenesis Intechopen



Frontiers Role Of The Il23 Il17 Pathway In Crohn S Disease Immunology
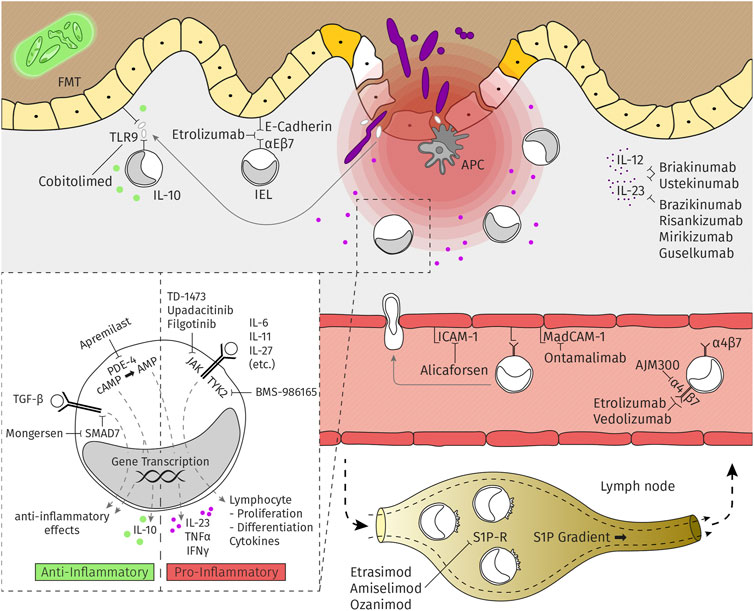



Frontiers An Update For Pharmacologists On New Treatment Options For Inflammatory Bowel Disease The Clinicians Perspective Pharmacology




Pdf Induction Therapy With The Selective Interleukin 23 Inhibitor Risankizumab In Patients With Moderate To Severe Crohn S Disease A Randomised Double Blind Placebo Controlled Phase 2 Study Semantic Scholar
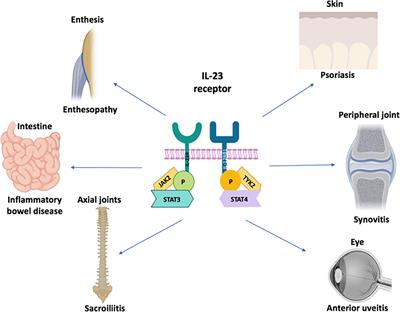



Frontiers Why Inhibition Of Il 23 Lacked Efficacy In Ankylosing Spondylitis Immunology
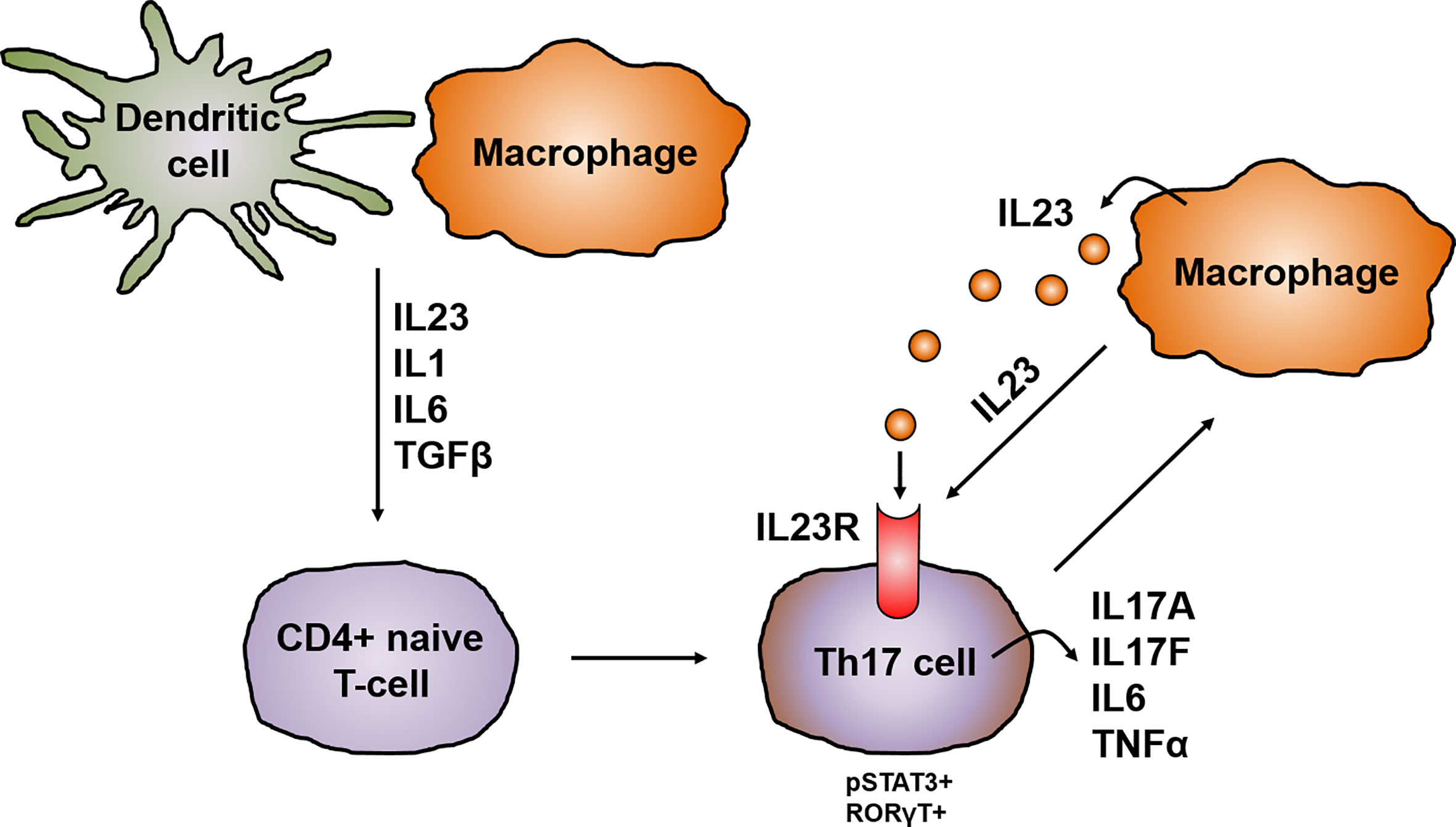



Frontiers Role Of The Il23 Il17 Pathway In Crohn S Disease Immunology




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect




A Role For Il 12 In Ibd After All Sciencedirect




Ulcerative Colitis Today Tomorrow And The Future European Medical Journal




Target Processes Or Pathways Of New Drugs With Evidence For Clinical Download Scientific Diagram



Inflammatory Bowel Disease Insight Report Current Therapies Drug Pipeline And Outlook Biospace




Rational Combination Therapy To Overcome The Plateau Of Drug Efficacy In Inflammatory Bowel Disease Gastroenterology




Classes Of Biologics For The Treatment Of Ibd Alpco




Classes Of Biologics For The Treatment Of Ibd Alpco
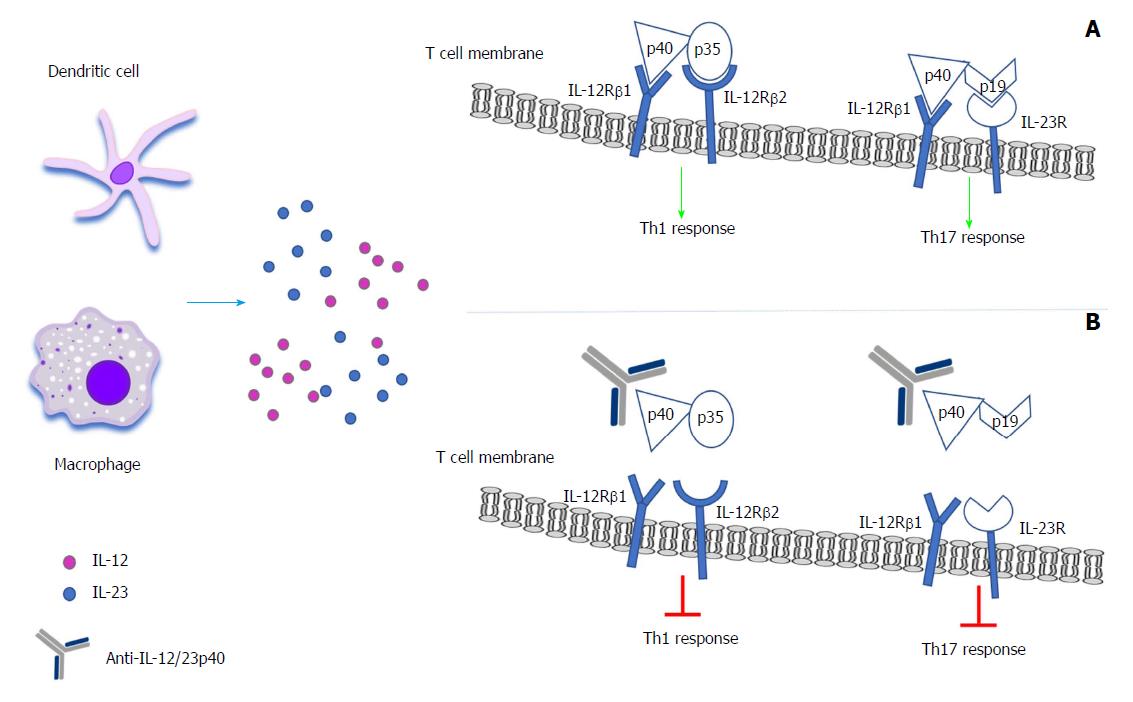



Interleukin 12 Interleukin 23 Pathway Biological Basis And Therapeutic Effect In Patients With Crohn S Disease




New Drugs In The Ulcerative Colitis Pipeline Prometheus Unbound Gastroenterology




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect
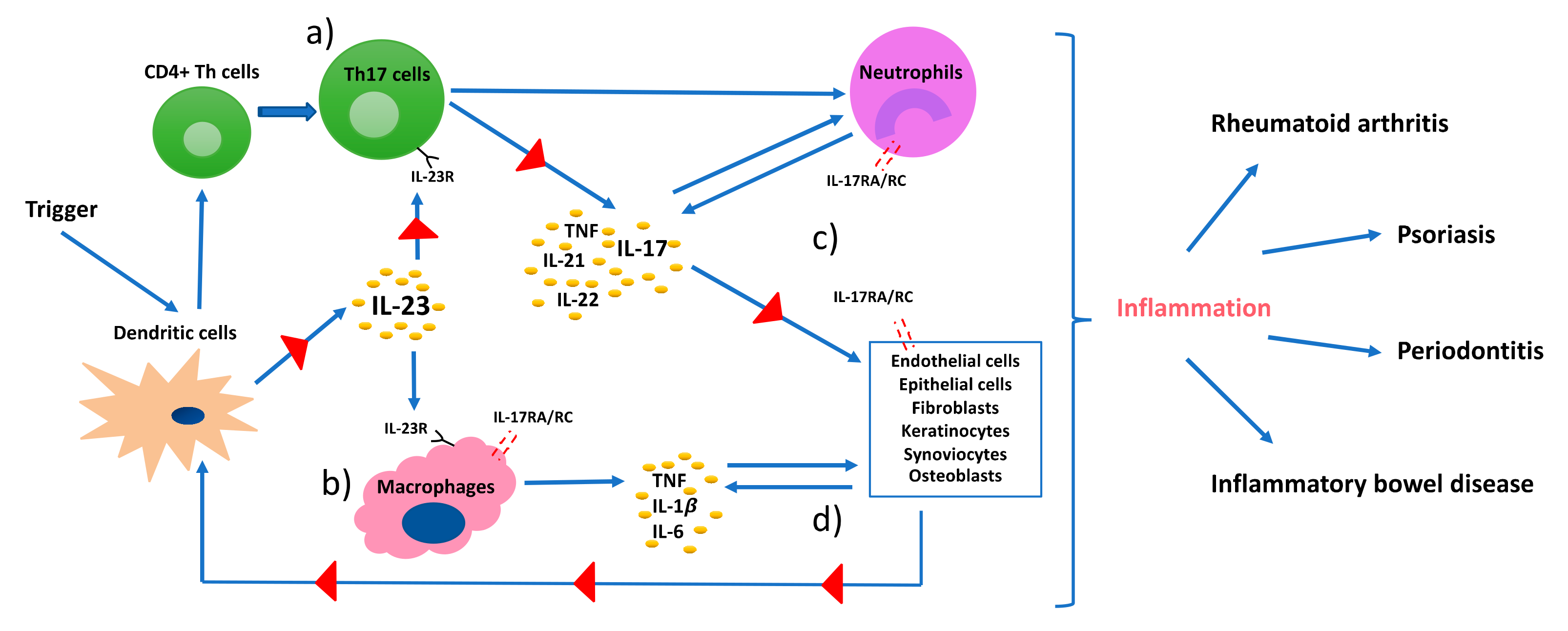



Ijms Free Full Text Th17 Cells And The Il 23 Il 17 Axis In The Pathogenesis Of Periodontitis And Immune Mediated Inflammatory Diseases Html




All Are Equal Some Are More Equal Targeting Il 12 And 23 In Ibd Nda Itt




Temporally Distinct Functions Of The Cytokines Il 12 And Il 23 Drive Chronic Colon Inflammation In Response To Intestinal Barrier Impairment Sciencedirect




Vitamin D Downregulates The Il 23 Receptor Pathway In Human Mucosal Group 3 Innate Lymphoid Cells Journal Of Allergy And Clinical Immunology




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology




Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut




Why Did Il 23p19 Inhibition Fail In As A Tale Of Tissues Trials Or Translation Annals Of The Rheumatic Diseases




Il23 Promotes Antimicrobial Pathways In Human Macrophages Which Are Reduced With The Ibd Protective Il23r R381q Variant Cellular And Molecular Gastroenterology And Hepatology
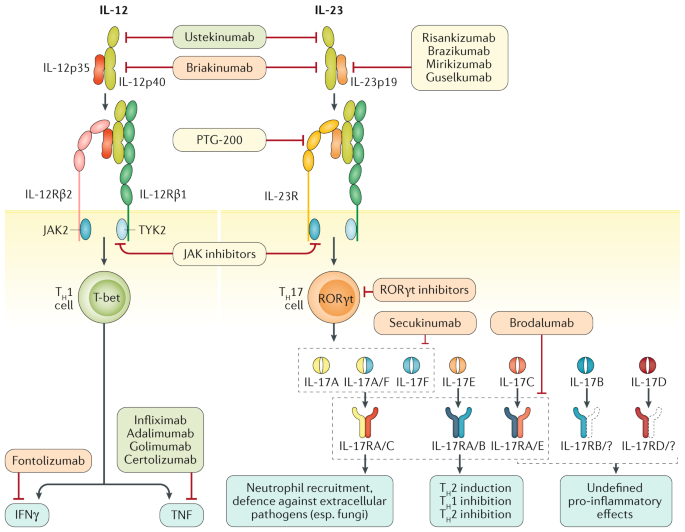



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology




Biologics Matching Drugs To Patients
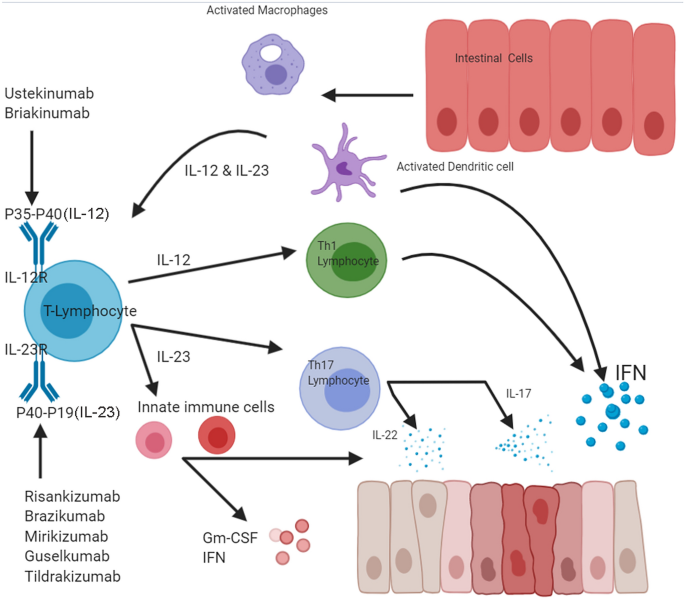



Clinical Trials Of Il 12 Il 23 Inhibitors In Inflammatory Bowel Disease Springerlink
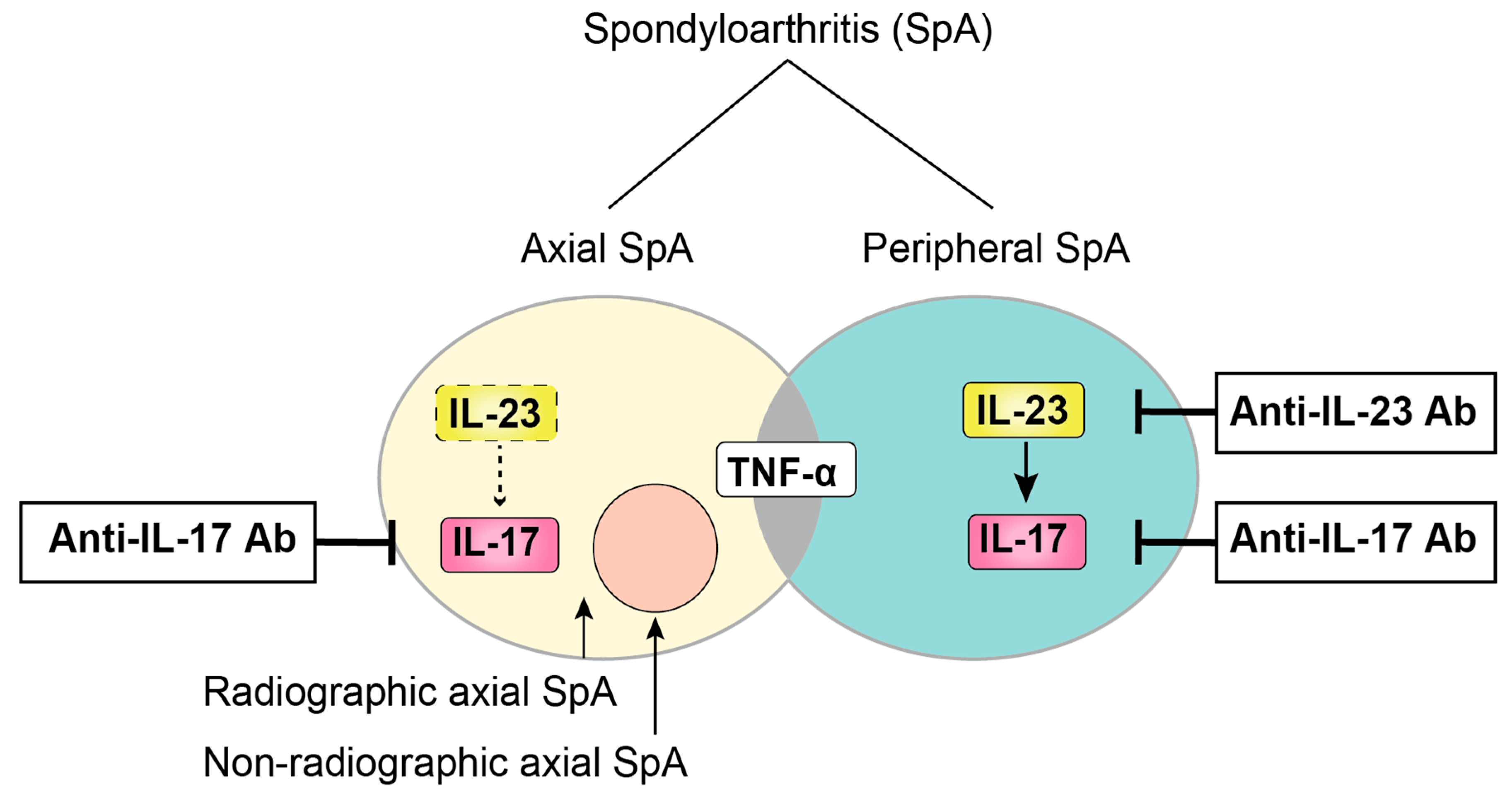



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html
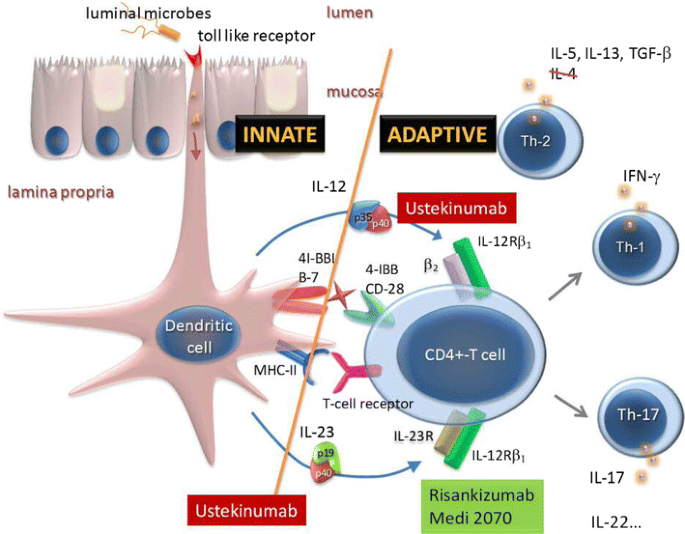



New Treatment Options For Inflammatory Bowel Diseases Springerlink
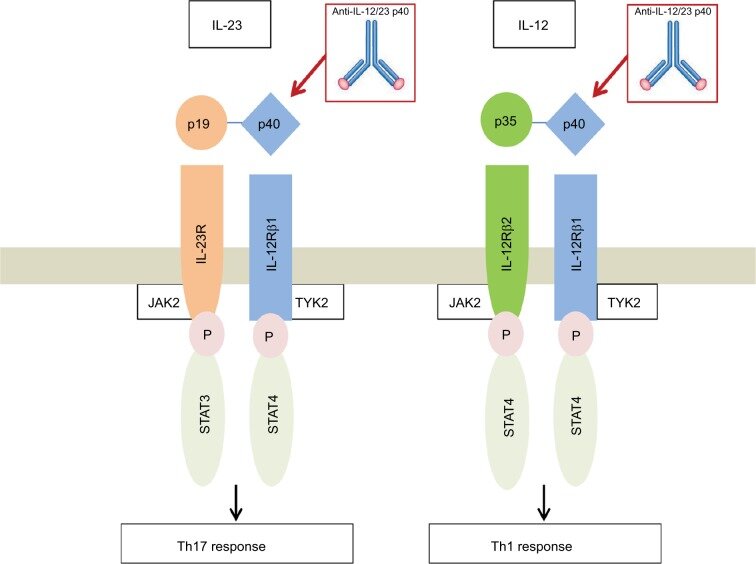



Schematic Representation Of Il 12 And Il 23 With Their Receptors And Download Scientific Diagram




Treating Challenging Moderate To Severe Crohn Disease With Il 12 Il 23 Inhibition Gastroenterology Advisor




Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Semantic Scholar
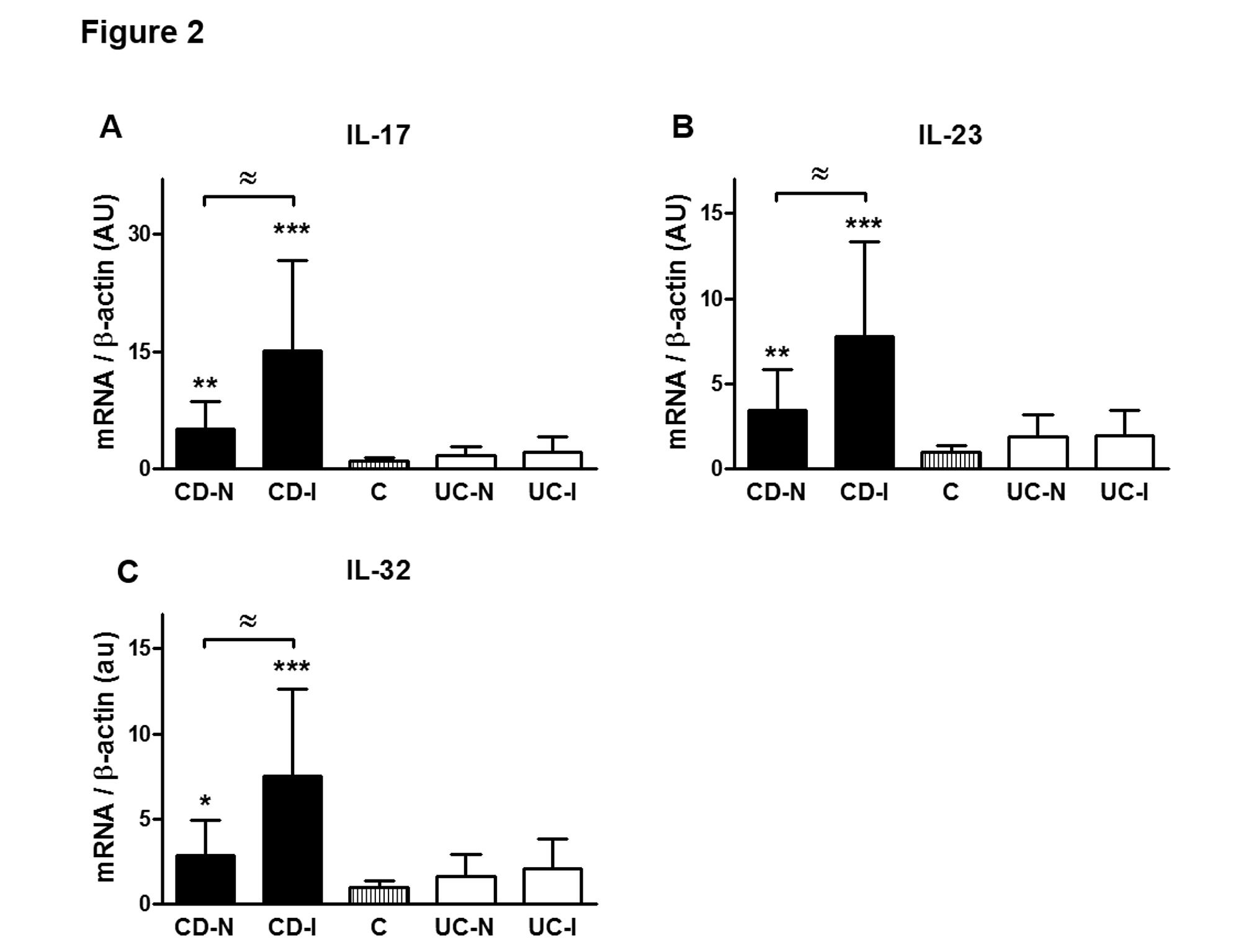



Cureus Crohn S Disease And Ulcerative Colitis Show Unique Cytokine Profiles
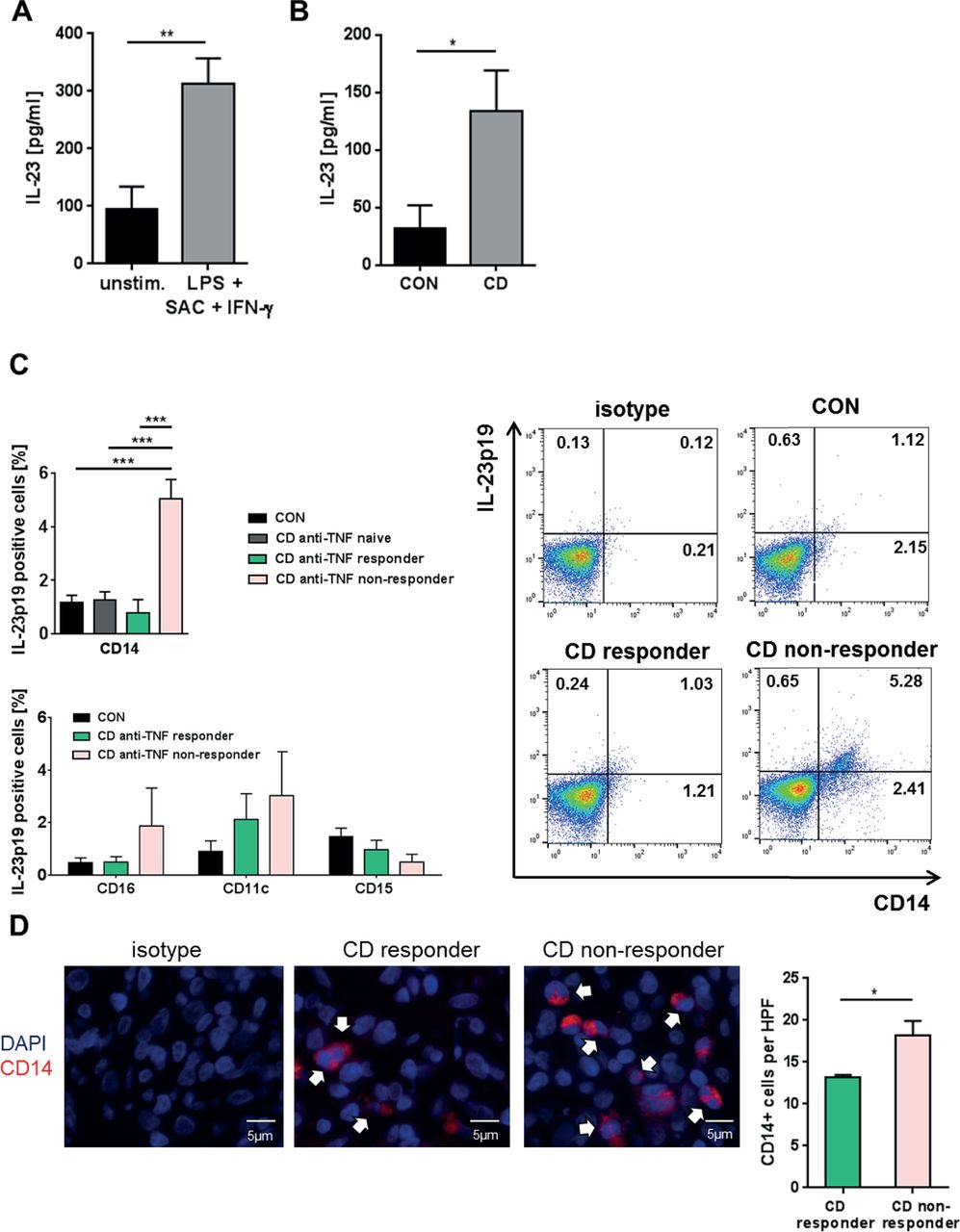



Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut




Il 23 In Inflammatory Bowel Diseases And Colon Cancer Sciencedirect
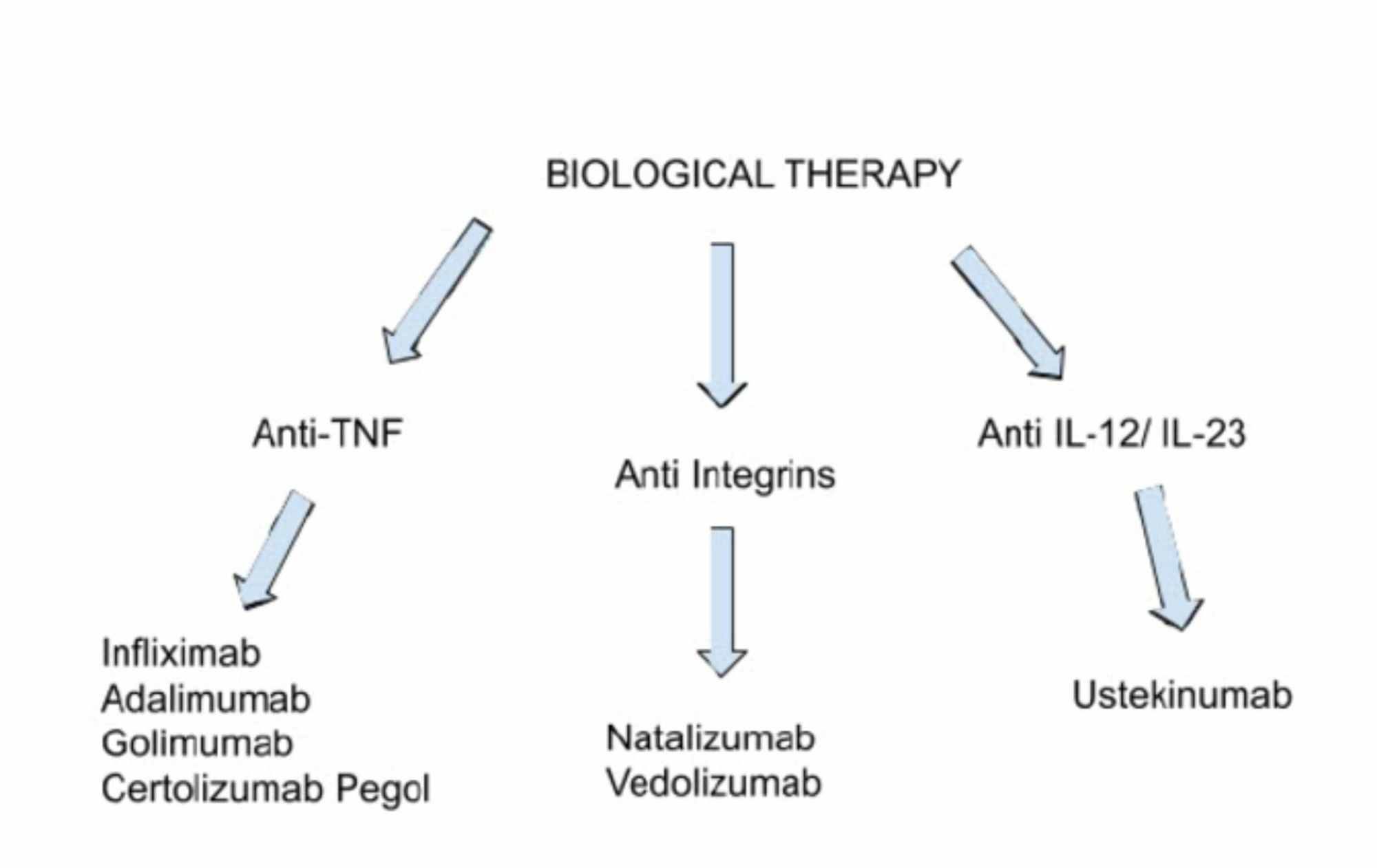



Cureus Biologics Targeting In The Treatment Of Inflammatory Bowel Disease A Conundrum




The Proinflammatory Effect Of Prostaglandin E2 In Experimental Inflammatory Bowel Disease Is Mediated Through The Il 23 Il 17 Axis The Journal Of Immunology




Optimizing Management Of Ibd Beyond Tnf A Inhibitors




Discovery Of The Il 23 Il 17 Signaling Pathway And The Treatment Of Psoriasis The Journal Of Immunology



2




Paradoxical Gastrointestinal Effects Of Interleukin 17 Blockers Annals Of The Rheumatic Diseases
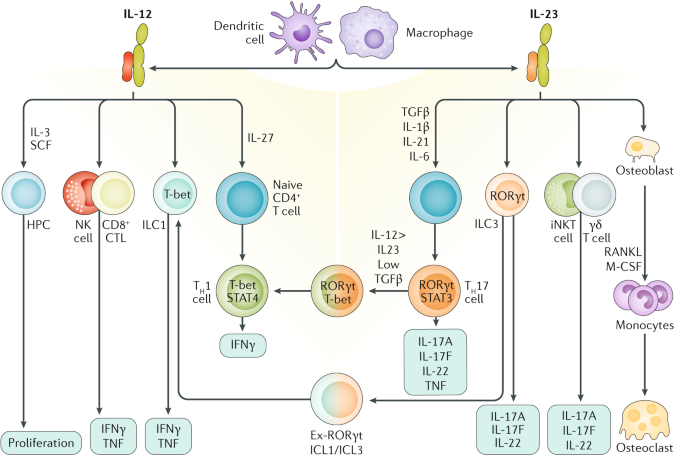



Il 12 Il 23 And Il 17 In Ibd Immunobiology And Therapeutic Targeting Nature Reviews Gastroenterology Hepatology
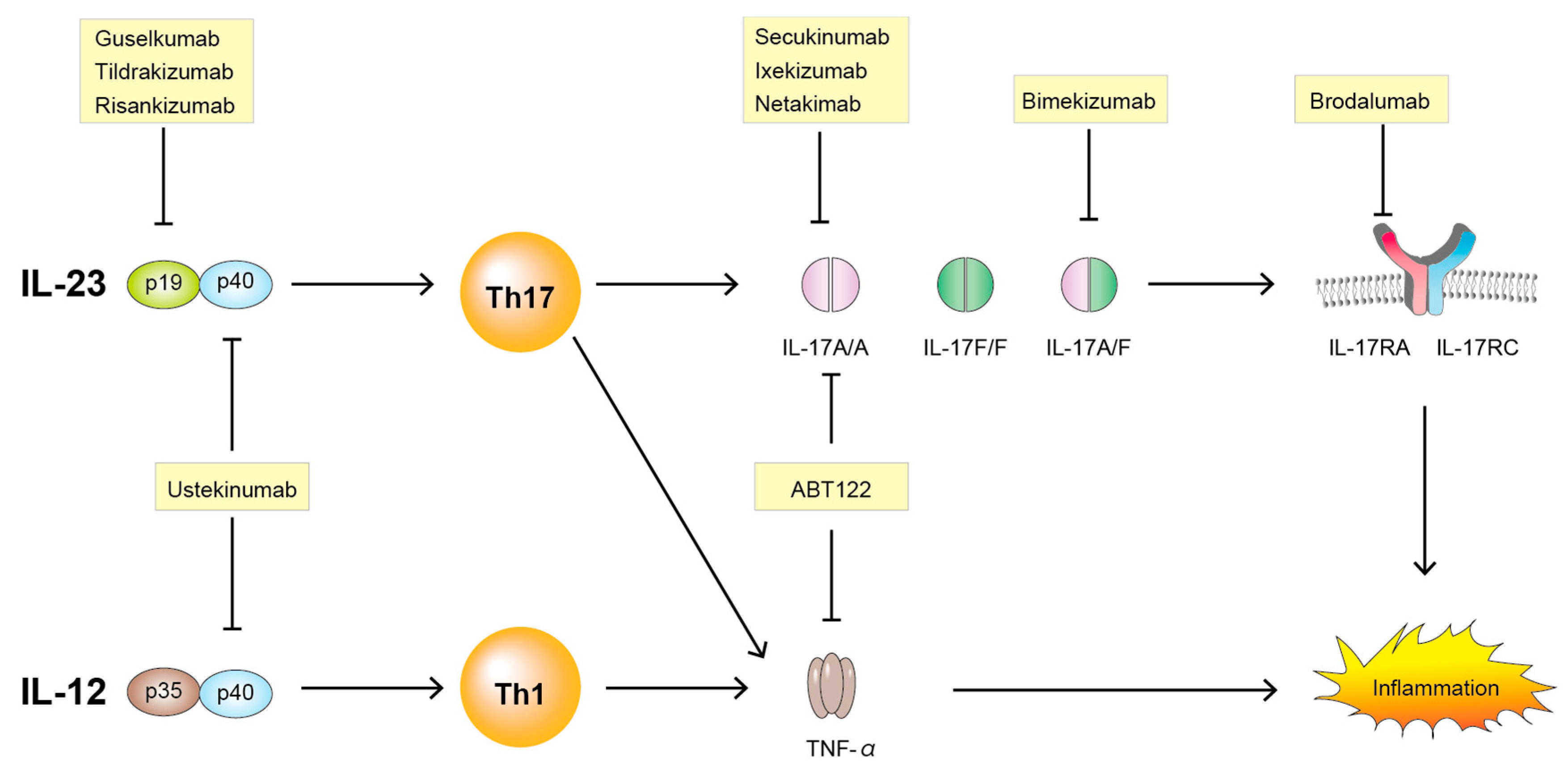



Ijms Free Full Text The Role Of The Il 23 Il 17 Pathway In The Pathogenesis Of Spondyloarthritis Html




Novel Pharmacological Approaches For Inflammatory Bowel Disease Targeting Key Intracellular Pathways And The Il 23 Il 17 Axis




Expansion Of Il 23 Receptor Bearing Tnfr2 T Cells Is Associated With Molecular Resistance To Anti Tnf Therapy In Crohn S Disease Gut



1




Targeting Interleukin 23 In The Treatment Of Noninfectious Uveitis Ophthalmology




Induction Therapy With The Selective Interleukin 23 Inhibitor Risankizumab In Patients With Moderate To Severe Crohn S Disease A Randomised Double Blind Placebo Controlled Phase 2 Study The Lancet




New Biologic Therapies That Target The Il 12 23 Pathway Youtube
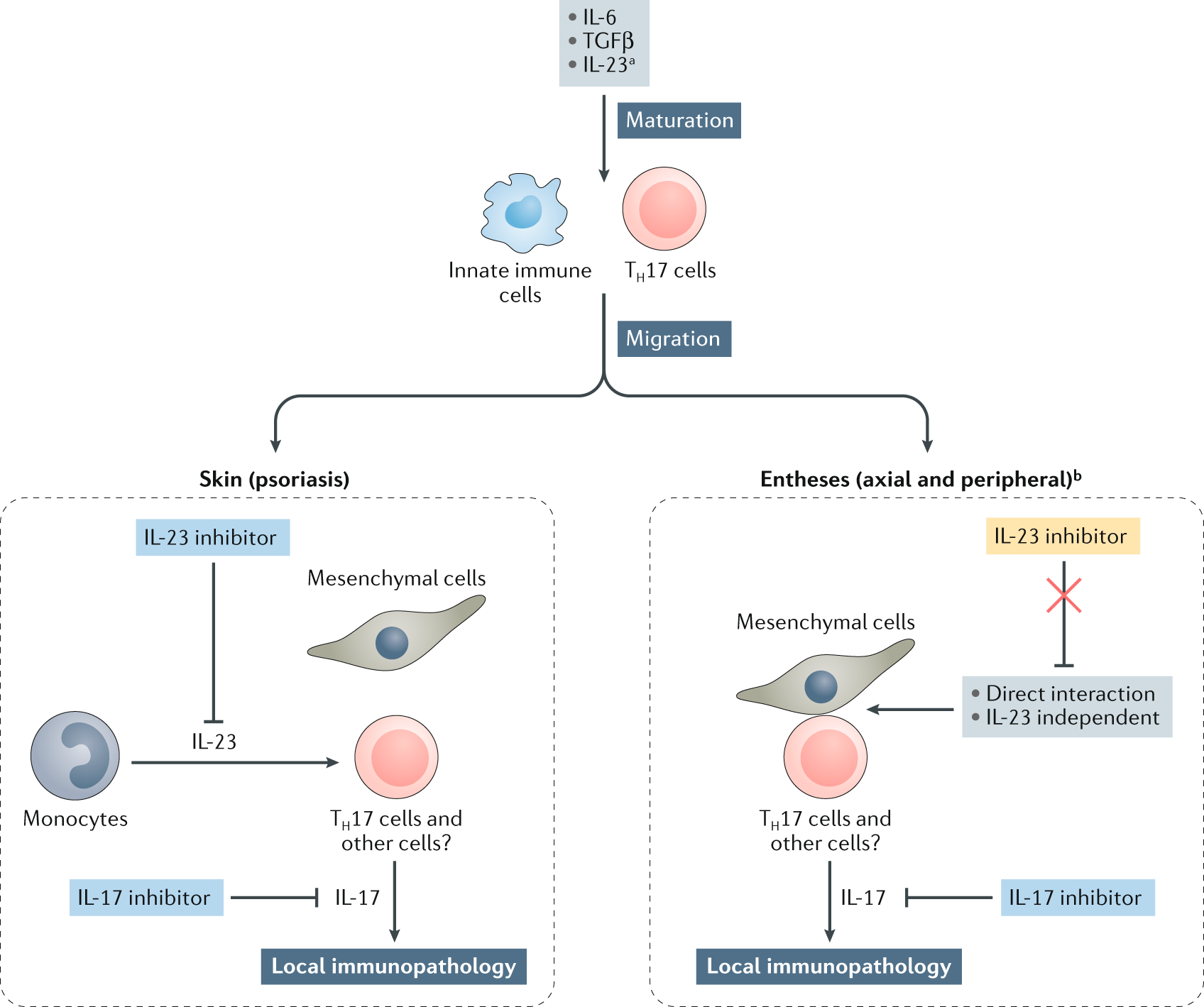



The Il 23 Il 17 Pathway As A Therapeutic Target In Axial Spondyloarthritis Nature Reviews Rheumatology



2
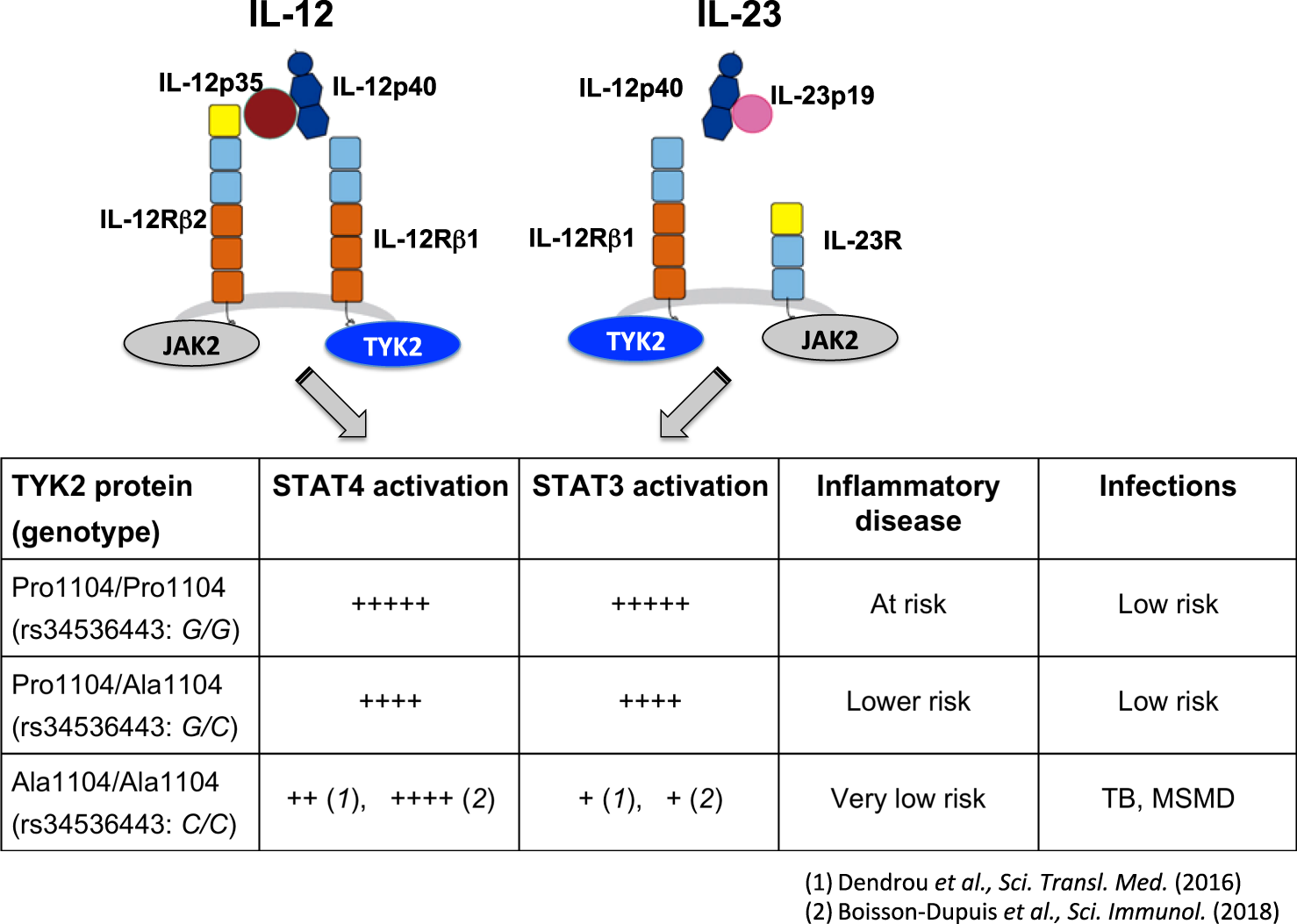



The Il 23 Il 17 Pathway In Human Chronic Inflammatory Diseases New Insight From Genetics And Targeted Therapies Genes Immunity



1
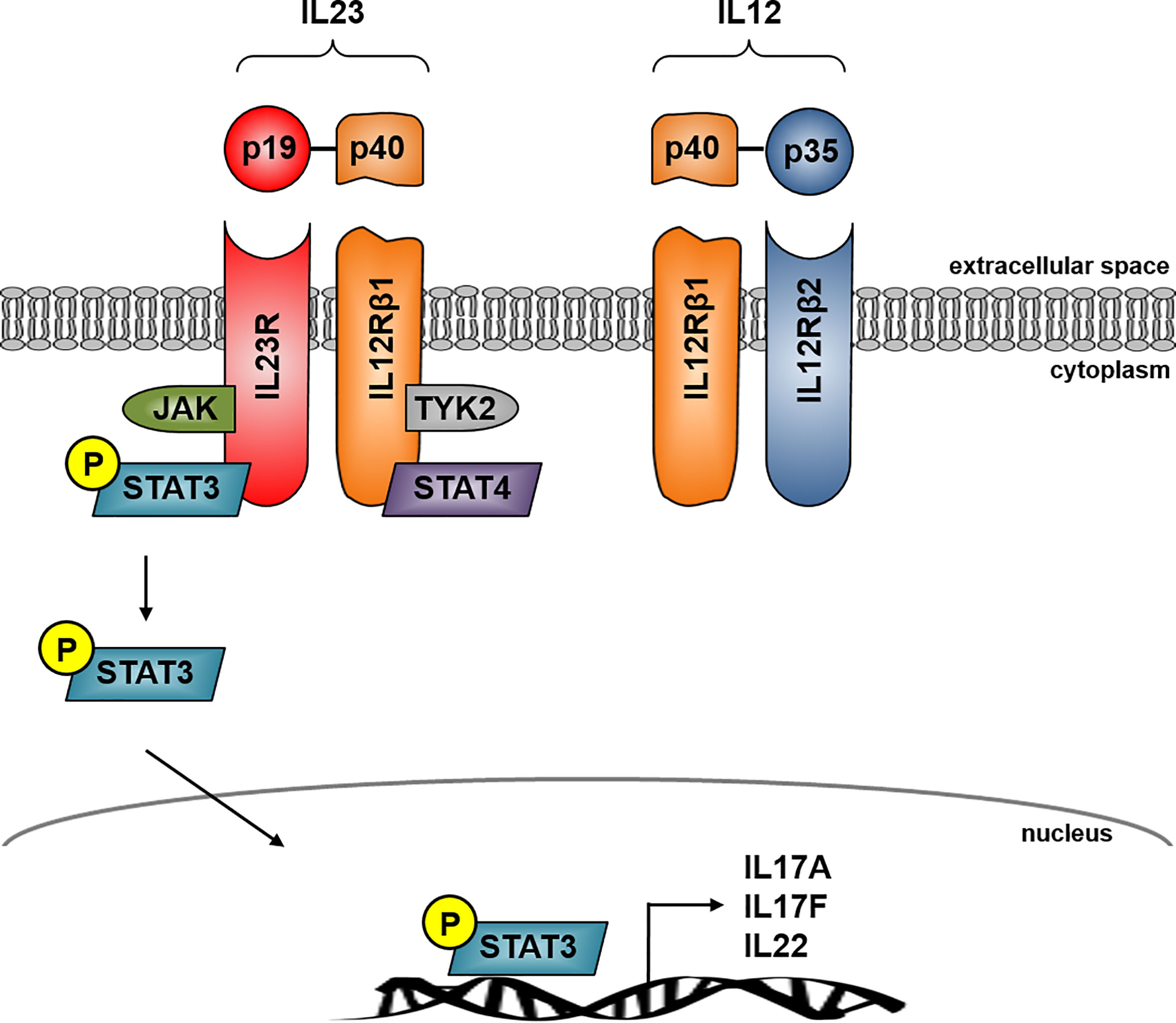



Frontiers Role Of The Il23 Il17 Pathway In Crohn S Disease Immunology




A Cytokine Network Involving Il 36g Il 23 And Il 22 Promotes Antimicrobial Defense And Recovery From Intestinal Barrier Damage Pnas




Classes Of Biologics For The Treatment Of Ibd Alpco



0 件のコメント:
コメントを投稿